Page last updated Oct 20, 2021 @ 10:14pm
How Solar Cells Work

How Solar Cells Work
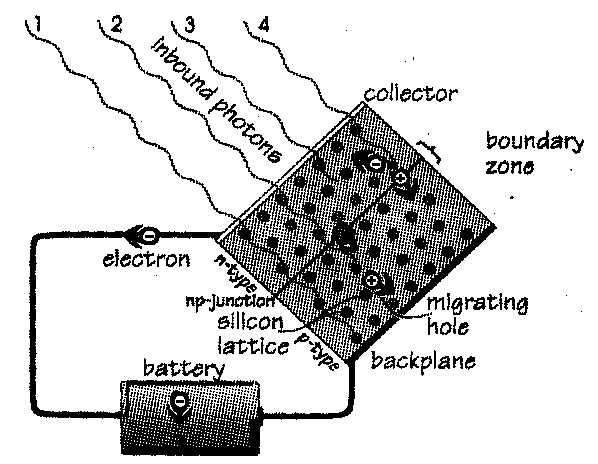
In the 1950s scientists tinkering with semiconductors found that by introducing small, minutely controlled amounts of certain impurities called dopants to the semiconductor matrix, the density of free electons could be shepherded and controlled. The dopants, similar enough in structure and valence to fit into the matrix, have one electron more or less than the semiconductor; for example, doping with phosphorus, which has five valence electrons, produces a (negative) n-type semiconductor, with an extra electron which can be dislodged easily. Aluminum, boron, indium, and gallium have only three valence electrons, and so a semiconductor doped with them is (positive) p-type, and has "holes" where the missing electrons ought to be. These holes behave just like electrons, except that they have an opposite, positive charge. (Holes are theoretical, but so are electrons, and either or both may or may not exist, but we know for sure that if one exists, they both do, because we can't create something out of nothing in the physical world.) It is important to understand that, although loosely bonded or extra carriers exist in a substance, it is still neutral electrically, because each atom's electrons are matched one for one by protons in the nucleus.
The fun begins when the two semiconductor types are intimately joined in a pn-junction, and the carriers are free to wander. Being of opposite charge, they move toward each other, and may cross the junction, depleting the region they came from, and transferring their charge to their new region. This produces an electric field, called gradient, which quickly reaches equilibrium with the force of attraction of excess carriers. This field becomes a permanent part of the device, a kind of slope that makes carriers tend to slide across the junction when they get close.
When light strikes a photovoltaic cell, atoms are bombarded with photons, and give up electrons. When an electron gets lopped off an atom, it leaves behind a hole, which has an equal and opposite charge. Both the electron, with its negative charge, and the hole, with its positive charge, begin a random walk generally down the gradient. If either carrier wanders across the junction, the field and the nature of the semiconductor material discourage it from recrossing. A proportion of carriers which cross this junction can be harvested by completing a circuit from a grid on the cell's surface to a collector on the backplane. In the cell, the light "pumps" electrons out one side of the cell, through the circuit, and back to the other side, energizing any electrical devices (like the battery in the diagram) found along the way.
This information was reprinted from The Independent Home by Michael Potts
Are YOU ready for the next power outage?
We welcome your feedback or questions. Click here to contact us.
See our Terms & Conditions before using information or
ordering from this web site.
Copyright © 1999-2025 NoOutage.com LLC. A Virginia LLC. All rights reserved.